Endolift Machine in Miami: Real vs Fake + Price & Safety Guide
Endolift Machine: Real vs Fake in Miami
In Miami, “Endolift” is a term you hear all the time. You see it on clinic menus, in Instagram captions, and in DMs asking about prices.
The problem is that not everything marketed as an “endolift machine” is the real thing. Some devices are just basic fiber lasers that use the name to follow the trend. The prices can vary a lot because the equipment is not the same.
This article is a practical, no-drama comparison between the Genuine EndoliftX® System and the types of “Endolift” look-alikes commonly sold online. We’ll break down the components of the real system, explain how to verify it, discuss why pricing varies so much, and outline what to check before you buy a device or book a treatment in Miami, Florida.
Why the word “Endolift” is a magnet for copycats
“Endolift” is sometimes used online as a generic label for endo-laser lifting devices (usually 1470nm and/or 980nm diode lasers with fibers). But EndoliftX® serves as a specific medical protocol that practitioners perform with specific instruments—not just any fiber laser. EndoliftX®’s official materials describe the system as a combination of a dedicated laser platform, certified microfibres, and official training.
Eufoton (the manufacturer behind the laser platform referenced by EndoliftX®) even addresses the confusion directly by distinguishing EndoliftX® from the so-called “Endolifting” performed with non-certified devices.
This same type of confusion also arises when patients compare different skin-tightening technologies without understanding the underlying systems and protocols, such as when deciding between Endolift and HIFU. A detailed comparison is explained here:
https://mynuceria.com/blog/endolift-vs-hifu-which-skin-tightening-treatment-is-better-for-you
Inside a genuine EndoliftX® setup: laser + microfibres + training
When people say “real Endolift machine,” the most useful way to think about it is:
It’s not one box. It’s a validated system + protocol.
EndoliftX® describes three tightly connected assets: laser, microfibres, and training.
The laser platform behind the protocol: LASEmaR® 1500
EndoliftX® states the laser used for treatments is LASEmaR® 1500 by Eufoton®, with software optimized for different face/body areas, energy dose adaptation, and even automatic recognition of fibers for each area.
According to Eufoton USA’s specifications, LASEmaR® 1500 is a 1470nm high-power diode (GaAs) system that includes a 635nm red aiming beam and uses air cooling combined with Peltier cells. Also described as portable (listed weight ~8.5 kg), it provides safety features such as software alerts and self-diagnostic capabilities that can stop emissions if anomalies occur.
Why that matters in Miami: you’re not only buying “a laser.” You’re purchasing repeatable outcomes, workflow, and safety architecture that your medical director (and your malpractice carrier) will value.
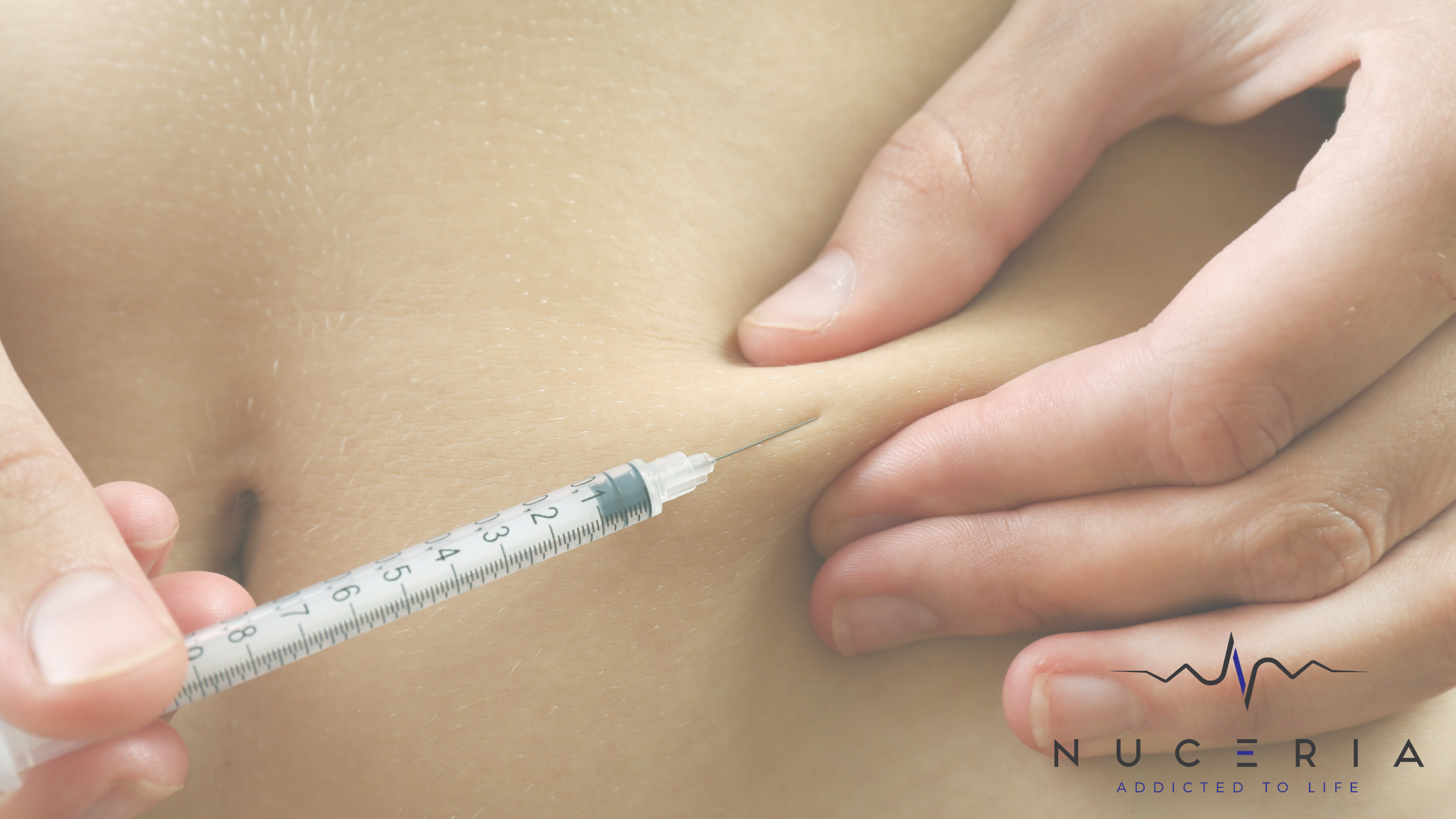
Single-use optical microfibres: sizes, coating, disposability
EndoliftX® emphasizes that its fibers are certified optical microfibres “as thin as hair,” invented by Eufoton and designed for interstitial use, with a specific coating intended to be light, ergonomic, and safe. It also specifies six calibers: 200 / 250 / 300 / 400 / 500 / 600 microns, and states that all microfibres are strictly disposable for patient safety.
Eufoton also describes the FTF (Fiber-to-Fiber) system as a setup that includes a power cable and a single-use, sterile optical fiber (30 cm), emphasizing “always new and sterile fiber,” “more safety,” and ease of use (dispose only the fiber tip after use).
This is one of the most significant practical differences between a verified system and a questionable one: with authentic systems, consumables, sterility, and traceability aren’t afterthoughts.
Training and accreditation: the part most knockoffs skip
EndoliftX® states doctors are carefully trained through the EndoliftX® Academy, and that only those who have received accreditation appear in the site’s “Find a Doctor” area.
For clinics, that training component is part of what you’re investing in. For patients, it’s part of how you verify you’re not walking into a “same name, different device” situation.
Why authenticity matters (and what we use at MyNuceria)
In Miami, people use “Endolift” as a catch-all term—and that’s precisely where confusion starts. Not every device advertised as an “endolift machine” is the real system.
At MyNuceria, we’re exceptionally clear about what we use: the EndoliftX® ecosystem, centered on the LASEmaR® 1500 (Eufoton®) and certified single-use optical microfibres. That combination is a big part of why patients choose us: it’s not about chasing trends—it’s about doing it the right way.
Patients looking for Endolift treatments in Miami performed with the EndoliftX® protocol can find full details here:
https://mynuceria.com/miami-fl/endolift/en
And because the experience should feel just as solid as the technology, Samantha Fonte supports patients throughout the process—making sure the plan is clear, personalized, and aligned with what you actually want to see in the mirror.
Want a quick authenticity check before you book anywhere?
“Are you using LASEmaR® 1500 for EndoliftX®?”
“Are the fibers certified and single-use?”
If a clinic can’t answer those clearly, that’s your sign to pause.
What a “fake” Endolift machine usually looks like online
Most “fake” scenarios aren’t perfect counterfeits with forged serial numbers. More commonly, you’ll see:
-
Generic fiber lasers are marketed using the word “Endolift.”
-
Claims like “Endolifting,” “Endolaser lift,” “Endolift machine,” with broad application lists
-
Unclear manufacturer identity, vague compliance language, and pricing that doesn’t match the verified ecosystem
A typical example is the listing you’ll find on significant marketplaces showing an “endolift laser machine price” in the $1,999–$4,999 range for “980nm/1470nm endolifting” devices.
For visual examples of how real vs look-alike Endolift machines are marketed online, see:
https://youtu.be/mjQ8_NZs8Vc
https://youtu.be/Wreekv-cThY
Common spec-sheet patterns that should trigger questions
If you’re comparing devices, here are patterns that often show up in non-verified listings:
-
“Dual wavelength 980nm + 1470nm” presented as interchangeable with EndoliftX®
-
Generic fibers (sometimes described as 200–600 μm) with no transparent single-use/sterile chain
-
“Certifications: FDA, CE, ISO, etc,” stated broadly without a clear documentation trail
To be clear: dual-wavelength lasers exist and can be legitimate products in their own right. The point is that “legitimate product” ≠ is the EndoliftX® system.” EndoliftX® explicitly frames EndoliftX® as performed exclusively with LASEmaR® 1500 plus optical microfibres and certified by notified bodies.
Absolute pricing vs too-good-to-be-true deals
Let’s talk about the part everyone Googles first: endolift machine price.
EndoliftX® states that the cost of Eufoton devices can vary depending on accessories and advises customers to request pricing via email, as they do not publicly list a fixed price.
That said, you can still triangulate reality by looking at the secondary market.
Typical market range for LASEmaR® 1500 (why it’s higher)
Used-equipment marketplaces commonly show LASEmaR® 1500 pricing in the five-figure range. For example:
-
Bimedis lists a range with a minimum of around $17.5k and a maximum of around $41k
-
Machinio shows a used listing at around $27,975
-
DOTmed shows a used listing at $41,000 (January 2026)
So if you see a “brand-new endolift laser machine price” advertised at a couple of thousand dollars, it may be a completely different product class than the EndoliftX® platform referenced by official channels.
Side-by-side comparison: genuine system vs look-alike listings
| What you’re comparing | Verified EndoliftX® ecosystem | Typical “Endolift” look-alike listing |
|---|---|---|
| Core setup | Laser + microfibres + training as one system | Often sold as a standalone device |
| Laser platform | LASEmaR® 1500 by Eufoton® | Generic 1470/980 diode lasers |
| Fibres | Certified optical microfibres, 200–600 μm, disposable | Fibres described, sterility often unclear |
| Safety architecture | Software alerts and self-diagnosis | Varies widely |
| Price signal | The five-figure used market is common | ~$1,999–$4,999 |
Final takeaway for Miami: don’t compare by name—compare by system
A genuine EndoliftX® experience is presented as a protocol delivered through a specific system (laser + certified disposable microfibres + training).
So whether you’re researching endolift machine, endolift machine price, endolift laser machine price, or hunting for an endolift machine for sale, your safest move is to verify what’s actually being sold (or used) before you decide based on a label—or a too-good-to-be-true number.
Request an appointment here: https://mynuceria.com or call Nuceria Health at (305) 398-4370 for an appointment in our Miami office.
Check out what others are saying about our services on Yelp: Wellness Center in Miami, FL.