Letybo®: South Korea’s #1 Botox
Letybo®: South Korea’s #1 Botox Alternative Receives FDA Approval
Letybo®: South Korea’s #1 Botox Alternative Receives FDA Approval and Launches in the U.S.
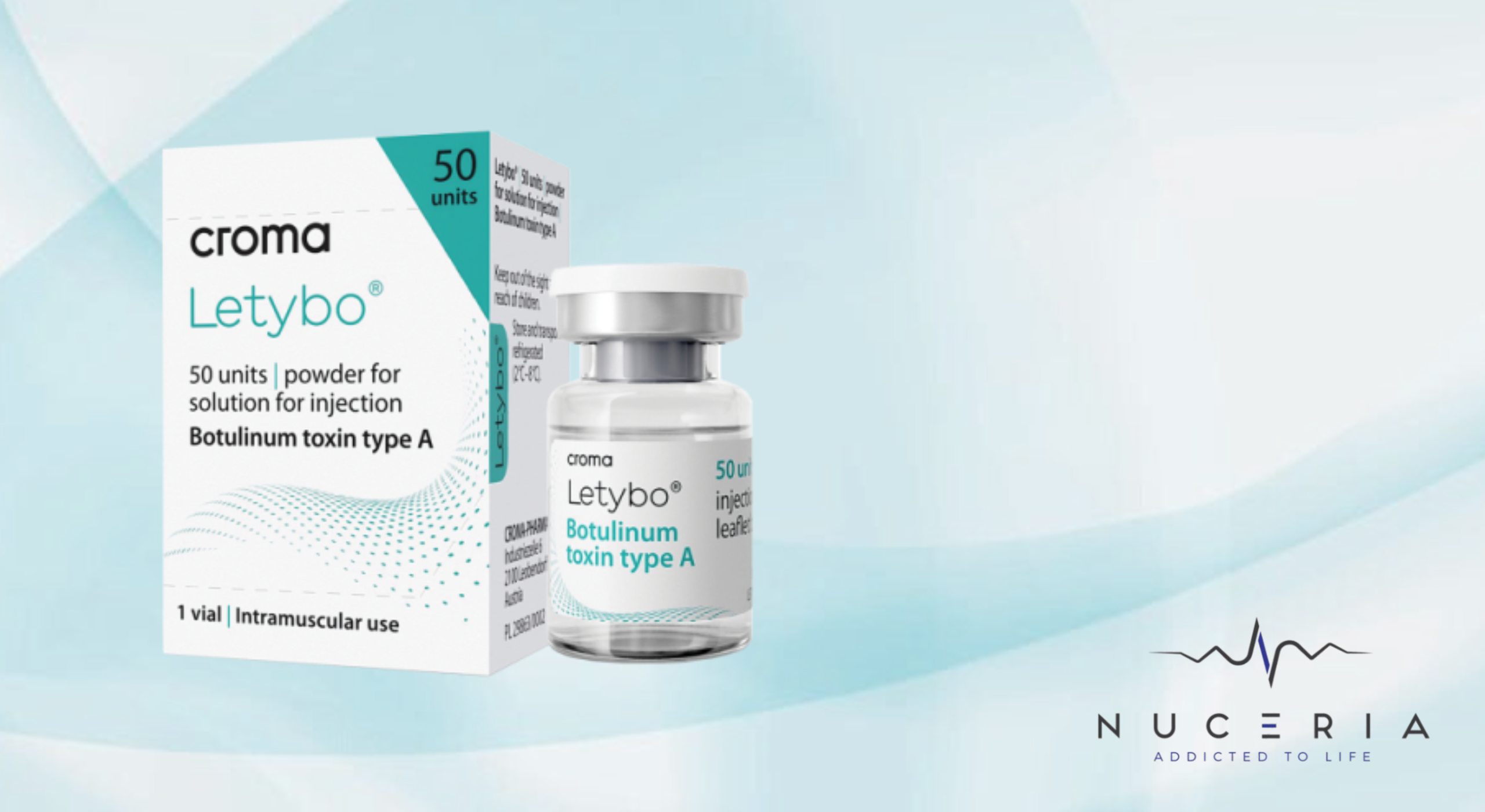
Miami, FL – [Date] – Hugel, in partnership with Croma-Pharma, announces the official FDA approval of Letybo®, South Korea’s leading botulinum toxin, now cleared for use in the treatment of glabellar lines in the United States. With this approval, Letybo® becomes the sixth botulinum toxin authorized in the U.S., joining the ranks of well-established aesthetic treatments and opening a new chapter in the global injectable market.
Already the top-selling botulinum toxin in Korea, Letybo® is now registered in 33 European countries and enters the U.S. backed by extensive clinical data, demonstrating a 94% response rate four weeks post-injection. Its active ingredient, letibotulinumtoxinA, offers high purity, proven safety, and consistent performance.
A Differentiated Approach to Wrinkle Reduction
What sets Letybo® apart from traditional neurotoxins like Botox®?
-
Rapid Onset: Visible improvements reported within just a few days post-treatment.
-
Extended Duration: Clinical trials show results lasting up to 16 weeks, with some patients experiencing effects for up to 6 months.
-
Natural-Looking Results: Designed to preserve facial expressiveness and avoid the “frozen look.”
-
Accessible Pricing: Letybo® is positioned as a cost-effective option without compromising quality.
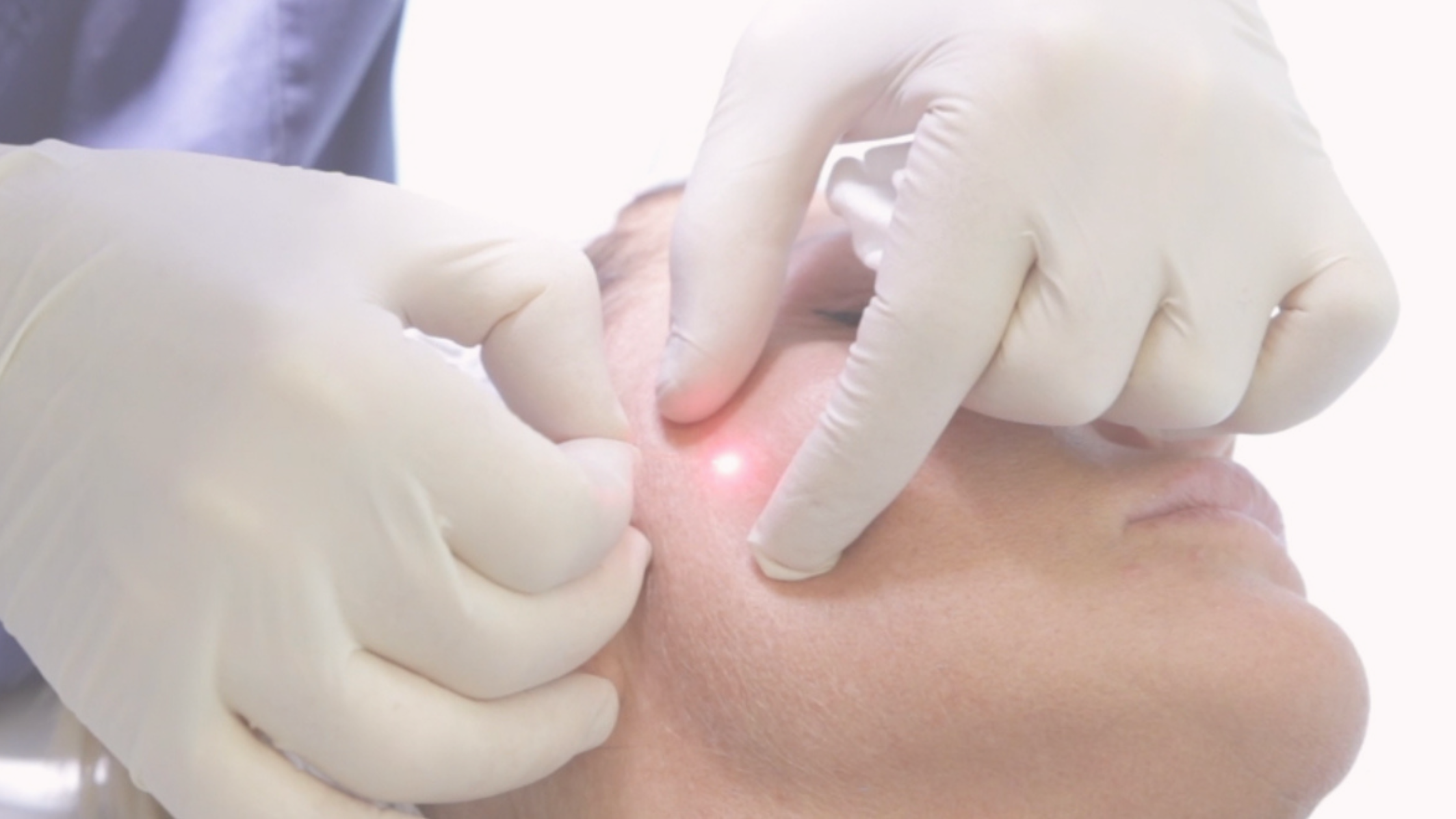
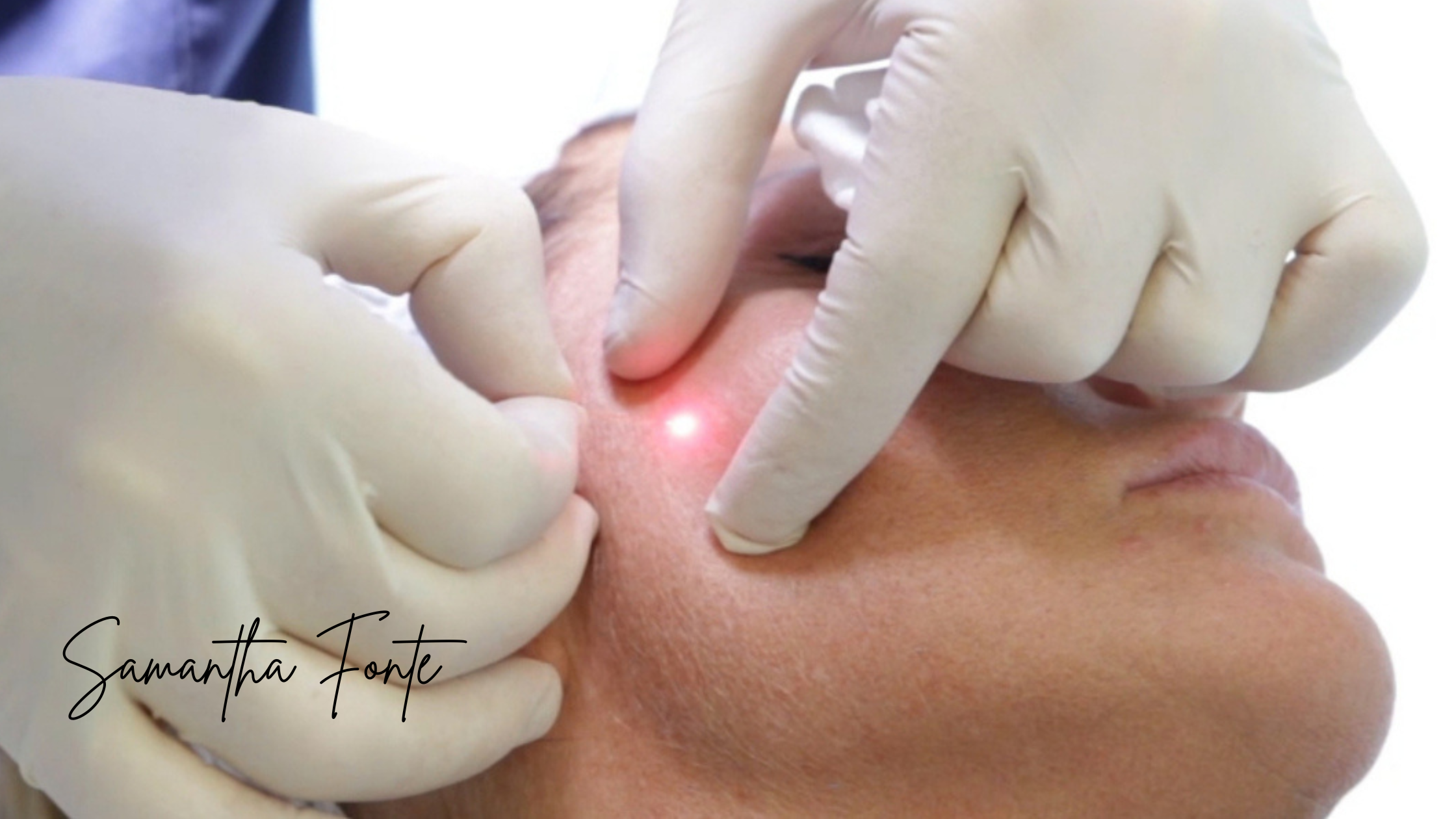
The product works by inhibiting the release of acetylcholine, temporarily relaxing facial muscles to reduce the appearance of dynamic wrinkles. This makes it an ideal solution for patients seeking minimally invasive, long-lasting facial rejuvenation.
Clinically Proven Results Backed by Extensive Studies
Letybo®’s efficacy has been confirmed through rigorous clinical research, including the Bless III study, which evaluated its performance in treating glabellar lines:
-
65% of patients achieved a composite score of “None” or “Mild” on the Glabellar Line Scale by Week 4.
-
79% improvement observed by investigator assessment, compared to 1% in the placebo group.
-
A 69% improvement was reported in patient self-assessment, compared to 0% with placebo.
-
The study met its primary endpoint, with a ≥2-point improvement from baseline.
These findings underscore Letybo®’s effectiveness and reinforce its credibility among medical professionals and patients.
U.S. Rollout and Availability
Letybo® will be available at select medical aesthetic clinics across the U.S., including Nuceria in Miami, a leading provider known for innovation and personalized aesthetic care. Medical professionals nationwide are being trained to deliver safe and effective results from day one.
About Hugel and Croma-Pharma
Hugel is a global biopharmaceutical company based in South Korea. It specializes in botulinum toxins, HA fillers, and skincare products. In partnership with Croma-Pharma, a European leader in minimally invasive aesthetics, Hugel continues to expand its international footprint by introducing Letybo® to key markets worldwide.
For media inquiries, clinical partnerships, or product information, please contact:
Request an appointment here: https://mynuceria.com or call Nuceria Health at (305) 398-4370 for an appointment in our Miami office.
Check out what others are saying about our services on Yelp: Wellness Center in Miami, FL.